Experimental and theoretical exploration of magnetic exchange interactions and single-molecule magnetic behaviour of bis(η1:η2:μ2-carboxylate)Gd III2/Dy III2 systems†
Abstract
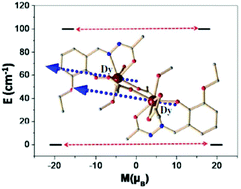
The present report deals with the syntheses, crystal structures, dc/ac magnetic properties and DFT/ab initio CASSCF calculations of two isomorphous bis(η1:η2:μ2-acetate)GdIII2/DyIII2 compounds of the formula [LnIII2L2(acetate)4(MeOH)2] (1, Ln = Gd; 2, Ln = Dy), where HL is (E)-N′-(3-ethoxysalicylidene)acetohydrazide. The two lanthanide(III) centres in each compound are symmetry-related owing to the presence of an inversion centre. Both compounds exhibit intramolecular ferromagnetic exchange interactions. The DyIII2 analogue is a single-molecule magnet (SMM) with Ueff = 52.8 cm−1 and τ0 = 1.52 × 10−6 s. DFT calculations for 1 and ab initio calculations for 2 also reveal ferromagnetic interactions. Ab initio calculations of the SMM behaviour of 2 and two other reported and structurally related compounds reveal the importance of the weak exchange interaction present between the two DyIII ions, and a relaxation mechanism has been developed to take into account the magnetic exchange interaction and to rationalize the observed difference in the Ueff values.


 Please wait while we load your content...
Please wait while we load your content...