Iron(iii)porphyrin electrocatalyzed enantioselective carbon-chloride bond cleavage of hexachlorocyclohexanes (HCHs): combined experimental investigation and theoretical calculations†
Abstract
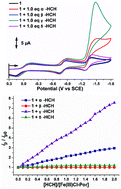
Enantioselective electrocatalysis of α-, β-, γ- and δ-hexachlorocyclohexanes (HCHs) by tetrakis-pentafluorophenyl-Fe(III)porphyrin is described. The first example of the combined use of electrochemical measurements and theoretical calculations to determine the mechanism of the enantioselective C–Cl bond cleavage of the electrocatalysis is reported. The electrochemical measurements demonstrate that the reactivity of the HCHs follows the order γ-HCH > α-HCH > δ-HCH > β-HCH. Steric considerations and a molecular orbital theory approach can be used to rationalize the enantioselective nature of the catalysis based on the ease of approach of each Cl atom to the central Fe(I) ion and a consideration of the nodes on the C–Cl bonds that weaken these bonds in a manner that results in bond cleavage and the formation of an Fe–Cl bond.


 Please wait while we load your content...
Please wait while we load your content...