Catalytic asymmetric synthesis of spirooxindoles: recent developments
Abstract
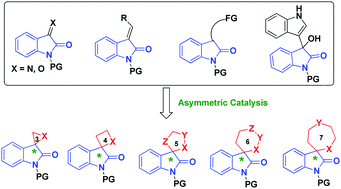
Chiral spirooxindoles are privileged heterocyclic motifs, which are widely found in natural alkaloids and pharmaceuticals. Moreover, the construction of chiral spiro-cyclic frameworks is a long-lasting challenge in organic synthesis. The past four years have witnessed significant developments in this field, and this feature article outlines the recent progress in the catalytic asymmetric synthesis of spirooxindoles, including the contributions of our group. The catalytic asymmetric construction of spirooxindoles has greatly benefited from the utilization of oxindole derivatives as starting materials, including isatin derivatives, methyleneindolinones, indolin-2-one derivatives and isatin-derived 3-indolylmethanols. This article is divided into sections according to the size and type of the generated spiro-ring fused at the C3-position of the oxindole core (from three-membered to seven-membered spiro-rings), and representative examples are given with illustrations. In addition, the enantioselective construction of bispirooxindole frameworks is also discussed in the last section.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...