Role of conformational dynamics in the evolution of novel enzyme function
Abstract
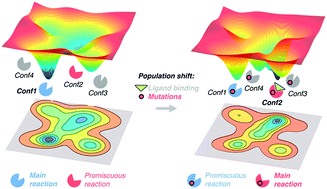
The free energy landscape concept that describes enzymes as an ensemble of differently populated conformational sub-states in dynamic equilibrium is key for evaluating enzyme activity, enantioselectivity, and specificity. Mutations introduced in the enzyme sequence can alter the populations of the pre-existing conformational states, thus strongly modifying the enzyme ability to accommodate alternative substrates, revert its enantiopreferences, and even increase the activity for some residual promiscuous reactions. In this feature article, we present an overview of the current experimental and computational strategies to explore the conformational free energy landscape of enzymes. We provide a series of recent publications that highlight the key role of conformational dynamics for the enzyme evolution towards new functions and substrates, and provide some perspectives on how conformational dynamism should be considered in future computational enzyme design protocols.
- This article is part of the themed collection: 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...