Rhodium-catalyzed asymmetric hydroboration of γ,δ-unsaturated amide derivatives: δ-borylated amides†
Abstract
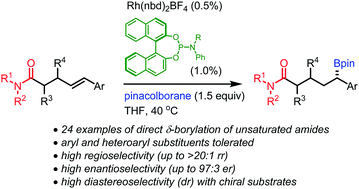
γ,δ-Unsaturated amides in which the alkene moiety bears an aryl or heteroaryl substituent undergo regioselective rhodium-catalyzed δ-borylation by pinacolborane to afford chiral secondary benzylic boronic esters. The results contrast the γ-borylation of γ,δ-unsaturated amides in which the disubstituted alkene moiety bears only alkyl substituents; the reversal in regiochemistry is coupled with a reversal in the sense of π-facial selectivity.


 Please wait while we load your content...
Please wait while we load your content...