Abstract
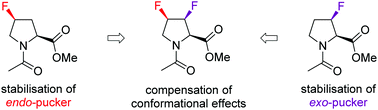
Monofluorination at the proline 4-position results in conformational effects, which is exploited for a range of applications. However, this conformational distortion is a hindrance when the natural proline conformation is important. Here we introduce (3S,4R)-3,4-difluoroproline, in which the individual fluorine atoms instil opposite conformational effects, as a suitable probe for fluorine NMR studies.
- This article is part of the themed collection: Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...