Invisible photochromism and optical anti-counterfeiting based on D–A type inverse diarylethene†
Abstract
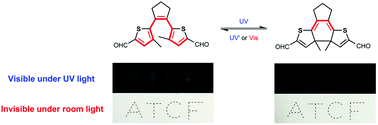
In most cases, inverse type diarylethenes (DAEs) exhibit a bathochromic shift of the π-conjugation absorption band after photocyclization, which leads to “visible” closed forms and “visible” photochromic behaviors. The reason for the bathochromic shift is that the lone pair electrons on the sulfur atoms of the thiophene rings function as π-electrons and participate in π-conjugation. To make photochromic behaviors “invisible”, electron-withdrawing aldehyde groups were introduced into the inverse DAE to endow lone pair electrons on the sulfur atoms with more n-electron characteristics and then achieved the hypsochromic shift of the π-conjugation absorption band after photocyclization. As a result, the absorption changes of aldehyde modified inverse DAE (IDAEo-2CHO) remained “invisible” during isomerization, and the only fluorescence changes can be observed by the naked eye after photocyclization. Taking advantage of this unique optical feature of IDAEo-2CHO, anti-counterfeiting applications were implemented.


 Please wait while we load your content...
Please wait while we load your content...