Site occupancy and luminescence properties of Ca3Ln(AlO)3(BO3)4:Ce3+,Tb3+,Mn2+ (Ln = Y, Gd)†
Abstract
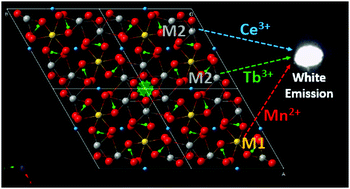
In this work, novel single-phase white emitting Ca3Ln(AlO)3(BO3)4:Ce3+,Tb3+,Mn2+ (Ln = Y and Gd) phosphors were synthesized by a conventional solid-state reaction method. The crystal structure and photoluminescence properties were investigated for the first time. The structure of Ca3Ln(AlO)3(BO3)4 was formed by AlO6 octahedral chains interconnected by BO3 triangles. Along the [001] direction, the Ca/Ln cations fill in the trigonal and hexagonal tunnels. For Ca3Ln(AlO)3(BO3)4:Ce3+ and Ca3Ln(AlO)3(BO3)4:Mn2+, large Ce3+ preferred to occupy the smaller coordination site, while small Mn2+ occupied the larger coordination site, which was explained by the space hindrance. Due to the cation disorder, the local environment of Ce3+ was complicated and the emission band was extremely broad. In Ca3Ln(AlO)3(BO3)4, the emission color can be tuned from blue to green or red by tuning the Ce3+–Tb3+ or Ce3+–Mn2+ concentration based on energy transfer. Thus, the combination of blue, green and red emitting Ce3+, Tb3+ and Mn2+ generated white emission.


 Please wait while we load your content...
Please wait while we load your content...