Pd-catalyzed asymmetric allylic alkylations via C–H activation of N-allyl imines with glycinates†
Abstract
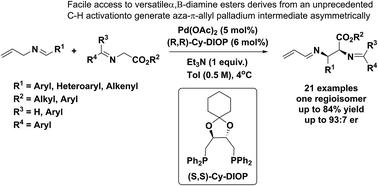
Herein is reported the first example of palladium-catalyzed asymmetric allylic alkylation (AAA) reactions involving 2-aza-π-allyl palladium intermediates. The 2-aza-π-allyl complex was generated via a novel mode of activation of N-allyl imines. Pd-catalyzed C(sp3)–H activation of N-allyl imines and subsequent nucleophilic attack by glycinates delivered vicinal diamino derivatives as the sole regioisomers with high levels of diastereo- and enantio-control in the presence of the chiral, bidentate (S,S)-Cy-DIOP ligand. This procedure is highly atom economical and could also be performed by a simple one-pot operation starting from aldehydes, allyl amines and glycinates under mild conditions. The products of this transformation could be converted into various useful derivatives, where the allyl substitution serves as a unique tool for differentiating the two amino moieties in the products.
- This article is part of the themed collection: Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...