Remote C–H insertion of vinyl cations leading to cyclopentenones†
Abstract
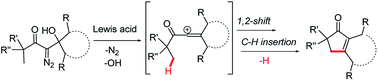
We report a Lewis acid catalyzed reaction sequence involving a 1,2-shift and subsequent C–H insertion that gives monocyclic and fused bicyclic cyclopentenone products. This reaction sequence, which is initiated by treating β-hydroxy-α-diazo ketones with a Lewis acid, proceeds through vinyl cation intermediates that insert at non-activated gamma C–H bonds. This reaction represents an alternative strategy to exploit the diazo functional group in an intramolecular C–H insertion, and can provide products not accessible by transition metal catalyzed C–H insertions. This remote C–H activation process provides good yields of bicyclic cyclopentenone products that contain 7- and 8-membered rings, and monocyclic prostaglandin analogs.


 Please wait while we load your content...
Please wait while we load your content...