Thio-Michael addition of α,β-unsaturated amides catalyzed by Nmm-based ionic liquids†
Abstract
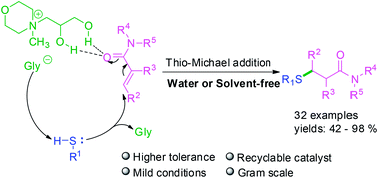
A simple and practical thio-Michael addition of α,β-unsaturated amides catalyzed by Nmm-based ionic liquids with a 1,2-propanediol group has been developed. All the α,β-unsaturated amides without substituents at the carbon end could smoothly react with sulfur-nucleophiles in water. Meanwhile for thio-Michael addition of α,β-unsaturated amides with substituents at the carbon end, the relevant product could also be obtained successfully under solvent-free conditions at 55 °C. Furthermore, the IL-catalyst is recyclable and applicable for gram-scale synthesis.


 Please wait while we load your content...
Please wait while we load your content...