Sequential asymmetric hydrogenation and photoredox chemistry with a single catalyst†
Abstract
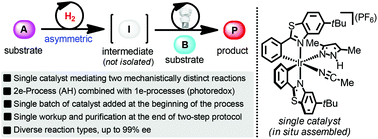
An important objective in organic synthesis is the generation of structural complexity in a straightforward and economical fashion. Herein, we address this challenge by reporting a process in which a single chiral iridium catalyst promotes two mechanistically distinct reaction types in a sequential fashion, namely asymmetric hydrogenation (two-electron mechanism) and photoredox chemistry (one-electron mechanism). A variety of chiral alcohols are generated with enantioselectivities of 91–99% ee using a single batch of catalyst added at the beginning of the reaction sequence without any requirement for isolating the reaction intermediates.


 Please wait while we load your content...
Please wait while we load your content...