Regioselective C5 alkenylation of 2-acylpyrroles via Pd(ii)-catalyzed C–H bond activation†
Abstract
A Pd(II)-catalyzed regioselective C5 alkenylation of 2-acylpyrroles has been developed employing 3-nitrile benzoyl group (N-(3-NCC6H4CO-)) as an efficient N-protecting group. The protocol provided a simple and efficient method to synthesize C5-alkenylated 2-acylpyrrole derivatives. The employed N-protecting group was readily removable in the presence of HCl/EtOH under mild conditions.
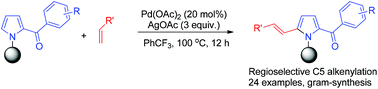
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...