Ruthenium-catalyzed meta-selective C–H sulfonation of azoarenes with arylsulfonyl chlorides†
Abstract
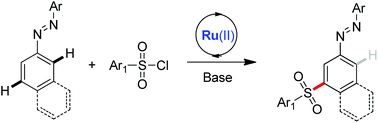
We have developed meta-selective CAr–H bond sulfonation of azoarenes with aryl sulfonyl chloride in the presence of the [Ru(p-cymene)Cl2]2 catalyst. Further reduction of the desired product provided a meta-sulfonated aromatic amine. Preliminary mechanistic studies indicated that the meta-sulfonation of azoarenes might involve an electrophilic aromatic substitution (SEAr) directed by the CAr–Ru σ-bond.


 Please wait while we load your content...
Please wait while we load your content...