Asymmetric total synthesis of (−)-δ-lycorane†
Abstract
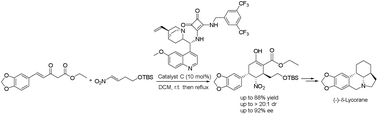
A first asymmetric synthesis of the lycorine-type Amaryllidaceae alkaloid (−)-δ-lycorane by using a chiral bifunctional squaramide-catalysed cascade reaction as a powerful tool to construct the skeleton of the alkaloid on the basis of unsaturated β-ketoester and nitroalkene is reported. The sequence afforded a highly functionalized intermediate with three stereogenic centres with high diastereoselectivity (>20 : 1 dr) and high enantioselectivity (92% ee) in one step, which was converted into (−)-δ-lycorane in eight steps.


 Please wait while we load your content...
Please wait while we load your content...