A subphthalocyanine–pyrene dyad: electron transfer and singlet oxygen generation†
Abstract
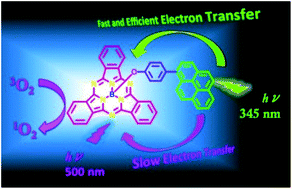
A light harvesting subphthalocyanine–pyrene dyad has been synthesized and characterized by linking pyrene (Py) with subphthalocyanine (SubPc) at its axial position with the B–O bond through the para position of the benzene group. Upon photoexcitation at the pyrene unit of the dyad, an efficient electron transfer from the singlet-excited state of Py to SubPc was observed. The electron transfer features were also observed by exciting the SubPc entity, but with slower rates (∼108 s−1). From the electrochemical measurements, the negative driving forces for charge separation via both the singlet states of Py and SubPc in the polar solvents indicate that the electron transfer is thermodynamically feasible. Interestingly, the examined compounds showed relatively high efficiency for producing the singlet oxygen (ΦΔ = ∼0.70). The collected data suggested the usefulness of the examined subphthalocyanine–pyrene dyad as a model of light harvesting system, as well as a sensitizer for photodynamic therapy.


 Please wait while we load your content...
Please wait while we load your content...