Addition of a polyhistidine tag alters the regioselectivity of carbonyl reductase S1 from Candida magnoliae†
Abstract
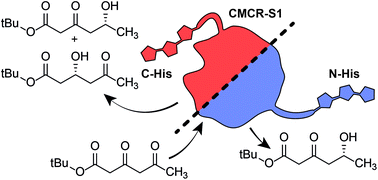
Studying enzymatic reductions of substrates with more than a single keto group is challenging, as the carbonyl reduction can create a vast array of regio- and stereoisomers. If used as reference compounds, regio- and stereopure hydroxy ketides could facilitate the characterization of reductases with unclear regio- and stereoselectivity. We have combined nonenzymatic and enzymatic reduction and oxidation steps to obtain all four regio- and stereoisomers of tert-butyl hydroxyoxohexanoates in high optical purity (enantiomeric ratio (er) of 99 : 1 for the δ-hydroxy-β-keto isomers; er of >97 : 3 for the β-hydroxy-δ-keto isomers). Furthermore, we have prepared seven of the eight possible regioisomers and diastereomers of γ-methylated hydroxyoxohexanoates. These 11 compounds allowed unraveling the complex stereoselectivity of β,δ-diketo ester reductions catalyzed by carbonyl reductase S1 from Candida magnoliae (CMCR-S1). Our analysis shows that the regio- and stereoselectivity of CMCR-S1-catalyzed reductions is highly sensitive toward modifications at the C-terminus of CMCR-S1: in addition to the expected δ-hydroxy product, the variant with a C-terminal His-tag also led to formation of β-hydroxy by-products with high optical purity.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...