A general strategy for the synthesis of indoloquinolizine alkaloids via a cyanide-catalyzed imino-Stetter reaction†
Abstract
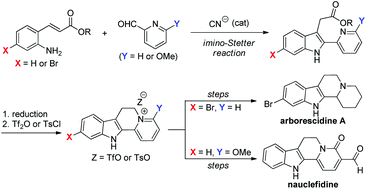
A new strategy applicable to the synthesis of indoloquinolizine natural products has been developed. A cyanide-catalyzed intramolecular imino-Stetter reaction of aldimines, derived from 2-aminocinnamic acid derivatives and 2-pyridinecarboxaldehydes, provided indole-3-acetic acid derivatives bearing a pyridyl ring at the 2-position. Reduction of the carboxylic acid moiety to an alcohol followed by activation of the resulting alcohol with Tf2O or TsCl generated indoloquinolizinium salts, which were utilized as precursors for indoloquinolizine natural products. The advantage of this protocol was successfully demonstrated in the total syntheses of arborescidine A and nauclefidine.


 Please wait while we load your content...
Please wait while we load your content...