Synthesis of kinase inhibitors containing a pentafluorosulfanyl moiety†
Abstract
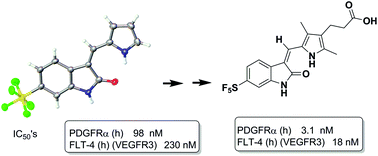
A series of 3-methylidene-1H-indol-2(3H)-ones substituted with a 5- or 6-pentafluorosulfanyl group has been synthesized by a Knoevenagel condensation reaction of SF5-substituted oxindoles with a range of aldehydes. The resulting products were characterized by X-ray crystallography studies and were tested for biological activity versus a panel of cell lines and protein kinases. Some exhibited single digit nM activity.
- This article is part of the themed collection: SBQ-RSC: Celebrating UK-Brazil collaborations


 Please wait while we load your content...
Please wait while we load your content...