Palladium-catalyzed tandem cyclization/sulfonylation of homoallenyl amides with sodium sulfinates†
Abstract
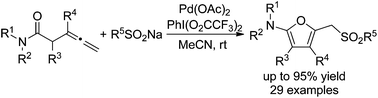
A palladium-catalyzed cyclizative sulfonylation of homoallenyl amides using sodium sulfinates as the sulfonylation reagent and PhI(O2CCF3)2 as the oxidant has been realized. The reaction proceeds at room temperature and produces structurally diverse 2-amino-5-sulfonylmethylfurans in good to excellent yields. A Pd(II)/Pd(IV) catalytic cycle has been proposed for the formation of sulfonylated furans. The concurrent formation of a furan moiety and a C(sp3)–sulfur bond in a single operation makes it a very attractive method for organic synthesis.


 Please wait while we load your content...
Please wait while we load your content...