Sigmatropic proton shifts: a quantum chemical study†
Abstract
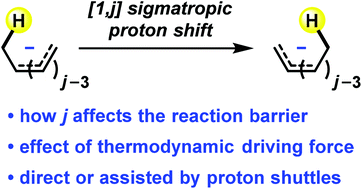
A quantum chemical study of [1,j] sigmatropic proton shifts in polyenyl anions and related conjugated systems has been performed. We found that the Woodward–Hoffmann rules can be applied to understand the stereochemical outcome of these sigmatropic rearrangements, showing that [1,j] sigmatropic proton shift occurs antarafacially when j = 4n + 2, while suprafacial proton shift is symmetry-allowed when j = 4n. The activation barriers for [1,j] proton shifts in polyenyl anions CjHj+3− are 48.2 (j = 2), 32.8 (j = 4), 21.0 (j = 6), 40.5 (j = 8), and 49.1 (j = 10) kcal mol−1, respectively. This trend can be explained by the trade-off between stereoelectronic requirement and ring strain in the proton shift transition structure. Among these reactions, only the [1,6] proton shift with the lowest activation barrier can occur intramolecularly under mild reaction conditions. The others are unlikely to take place in a direct manner. Consequently, proton shuttles are generally required to facilitate these sigmatropic proton shifts through a protonation/deprotonation mechanism.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...