Synthesis of the octahydronaphthalene core of nahuoic acid A via a B(C6F5)3-catalyzed intramolecular Diels–Alder (IMDA) reaction†
Abstract
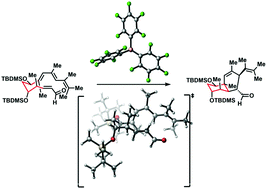
Model tetraenal 9b underwent intramolecular Diels–Alder cycloaddition in CH2Cl2 at −10 °C under catalysis by the bulky Lewis acid B(C6F5)3 to deliver as major components the cis-fused angularly-methylated octahydronaphthalene products, which are formed through the alternative exo orientations of the reacting moieties. One of these diastereomers features the relative and absolute configuration present in the core of nahuoic acid A, a natural product that acts as a cofactor-competitive inhibitor of the lysine methyl transferase SETD8. By contrast, catalysis of the reaction by Me2AlCl at −40 °C selectively afforded the trans-fused isomer resulting from the Re-endo orientation.


 Please wait while we load your content...
Please wait while we load your content...