Strained alkynes derived from 2,2′-dihydroxy-1,1′-biaryls; synthesis and copper-free cycloaddition with azides†
Abstract
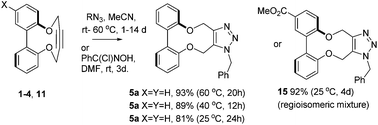
A series of strained alkynes were prepared from 2,2′-dihydroxy-biaryls. Several were characterised by X-ray crystallography, revealing strained C(sp)–C(sp)–C(sp3) bond angles in the range of 163–167°. Their cycloadditions with azides proceed without a catalyst. Functionalised versions of these reagents have potential applications to materials synthesis and bioconjugations.


 Please wait while we load your content...
Please wait while we load your content...