Metal-free phosphonation of heteroarene N-oxides with trialkyl phosphite at room temperature†
Abstract
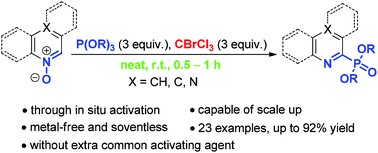
A new protocol is described for the conversion of heteroarene N-oxides to heteroarylphosphonates through in situ activation with bromotrichloromethane. The N-oxides of isoquinoline, quinoline, quinoxaline and 1,10-phenanthroline were fast transformed into the corresponding heteroarylphosphonates in up to 92% yield under mild conditions in the absence of solvent and metal catalysts. The good functional group tolerance, low cost, feasibility of scale up, and wide availability of reagents make this method a prominent complement to the Hirao coupling.


 Please wait while we load your content...
Please wait while we load your content...