A target oriented expeditious approach towards synthesis of certain bacterial rare sugar derivatives†
Abstract
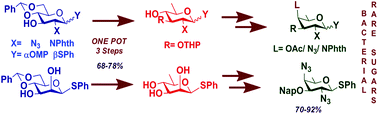
Bacterial rare amino deoxy sugars are found in the cell surface polysaccharides of multiple pathogenic bacterial strains, but are absent in the human metabolism. This helps in the differentiation between pathogens and host cells which can be exploited for target specific drug discovery and carbohydrate based vaccine development. The principal bacterial atypical sugar derivatives include 2-acetamido-4-amino-2,4,6-trideoxy-D-galactose (AAT), 2,4-diacetamido-2,4,6-trideoxy-D-galactose (DATDG) and N-acetylfucosamine (FucNAc). Herein, a highly streamlined protocol leading to the aforesaid derivatives is presented. The highlights of the method lie in radical mediated 6-deoxygenation along with a one-pot like protection profile manipulation on suitably derivatised D-glucosamine or D-mannose motifs to obtain a vital quinovosaminoside or rhamnoside from which rare sugar derivatives were synthesized in a diversity oriented manner.


 Please wait while we load your content...
Please wait while we load your content...