Studies on cyclization reactions of 3-amino-2,4-dihydroxybutanoic acid derivatives†
Abstract
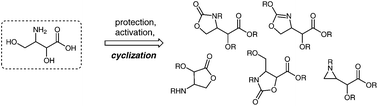
A number of cyclic derivatives of 3-amino-2,4-dihydroxybutanoic acid are known in the literature but they are often prepared from other cyclic precursors. This study showed that the title compound too may serve as a convenient substrate for cyclization reactions. Using orthogonally-protected linear derivatives, regioselective cyclizations were performed, leading to original and highly-functionalized γ-lactones, oxazolidinones, oxazolines and aziridines. In these reactions a key role was played by the C3 nitrogen group function, while the C2 alcohol function showed no propensity for participation in cyclization reactions.


 Please wait while we load your content...
Please wait while we load your content...