Controlling biomineralisation with cations
Abstract
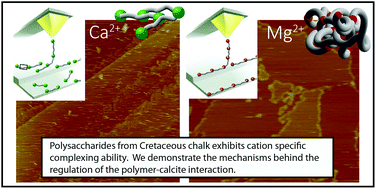
The production of polymers for controlling calcite growth is a well-known approach in biomineralising organisms. Numerous studies have shown that polymers significantly influenced the growth rate and morphology of CaCO3 but little is known about how the polymers are actually controlled by the organisms. Here we show that cations control the effect of polysaccharides and that these processes have been in place for at least 60 million years. We studied the interaction between cleaved samples of pure calcite and ancient coccolith associated polysaccharides (aPS) that we had extracted from the samples of Cretaceous chalk, in solutions that contained one of the common seawater cations, K+, Ca2+, Mg2+ and Sr2+. With atomic and chemical force microscopy (AFM and CFM), we showed that K+, Ca2+ and Sr2+ complex aPS through a weak, outer sphere bonding, giving the aPS affinity to sites on steps and terraces. In contrast, Mg2+ enhanced the formation of stronger and longer aPS complexes, resulting in low affinity to calcite terraces and strong affinity to steps. It is known that adsorption is influenced by ionic potential and ionic strength. Our results show that cation–polysaccharide complexing can modify the effectiveness of the polymer. Thus, creating organic molecules with cation complexing ability is an effective strategy for regulating mineral growth, both now and in the past.


 Please wait while we load your content...
Please wait while we load your content...