Ultrathin TiO2-B nanowires as an anode material for Mg-ion batteries based on a surface Mg storage mechanism†
Abstract
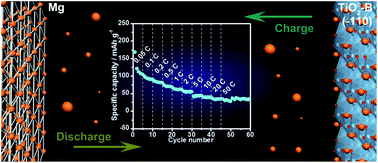
Ultrathin TiO2-B nanowires with a naked (−110) surface were prepared by a hydrothermal process and used as the anode material for Mg-ion batteries. The material delivered a reversible Mg2+ ion capacity of 110 mA h g−1 at the 0.1C rate. Excellent cycling stability was achieved with a small capacity-fading rate of 0.08% per cycle. In addition, a discharge capacity of 34 mA h g−1 was obtained at the 50C rate, demonstrating the material's excellent high rate capability. First-principles calculations showed that Mg2+ ions hardly penetrated into the TiO2-B lattice because of a very large Mg2+ ion diffusion barrier of 0.63 eV. Instead, the Mg2+ ions were stored at the 4-coordinated vacancies of TiO2-B nanowire (−110) surfaces. The adsorbed Mg2+ ions were bonded with unpaired surface oxygen atoms. Meanwhile, a small amount of electrons were transferred from the O-2p state to the Ti-3d state.


 Please wait while we load your content...
Please wait while we load your content...