Et3GeH versus Et3SiH: controlling reaction pathways in catalytic C–F bond activations at a nanoscopic aluminum chlorofluoride†
Abstract
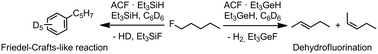
Catalytic C–F bond activation reactions of mono- and polyfluoroalkanes at Lewis acidic amorphous aluminum chlorofluoride (ACF) are presented. The hydrogen sources Et3GeH or Et3SiH control the selectivity of the conversions. The immobilization of Et3GeH at ACF resulted in catalytic dehydrohalogenation reactions to yield olefins under very mild conditions. In contrast, if Et3SiH is immobilized at ACF, C–C coupling occured and the formation of Friedel–Crafts products was observed. MAS NMR spectroscopic studies revealed information about the surface binding of the substrates.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...