White phosphorus derived PdAu–P ternary alloy for efficient methanol electrooxidation†
Abstract
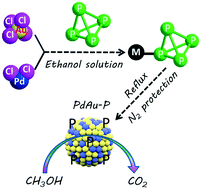
Metal phosphides (M–P) have recently emerged as highly active, low-cost alternatives to noble metals for all kinds of electrocatalytic reactions. However, the synthesis of M–P catalysts with high P contents is commonly laborious and involves multiple synthetic steps. Herein, a facile and straightforward white phosphorus (P4) derived reduction method is reported to fabricate ternary palladium–gold–phosphorus (PdAu–P) electrocatalysts. The resultant PdAu–P sample shows outstanding electrocatalytic activity (specific and mass activity), long-term stability and enhanced COads anti-poisoning ability compared with its counterparts, including the PdAu sample and the commercial Pd black catalyst for methanol oxidation reaction (MOR) in alkaline medium. Structural advantages (high P contents and small particle-sizes) and potential synergistic effects (electronic and geometric effect) among various elements (Pd, Au and P) contribute to such impressive MOR performance of the ternary PdAu–P electrocatalyst. The synthetic strategy presented here offers a promising platform for the fundamental studies of the fabrication of both metal phosphides with high P contents and high-performance electrocatalysts for applications in direct methanol fuel cells (DMFCs).


 Please wait while we load your content...
Please wait while we load your content...