Deciphering environment effects in peptide bond solvation dynamics by experiment and theory†
Abstract
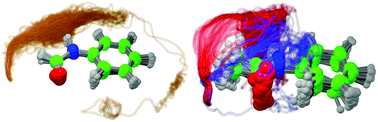
Most proteins work in aqueous solution and the interaction with water strongly affects their structure and function. However, experimentally the motion of a specific single water molecule is difficult to trace by conventional methods, because they average over the heterogeneous solvation structure of bulk water surrounding the protein. Here, we provide a detailed atomistic picture of the water rearrangement dynamics around the –CONH– peptide linkage in the two model systems formanilide and acetanilide, which simply differ by the presence of a methyl group at the peptide linkage. The combination of picosecond pump–probe time-resolved infrared spectroscopy and molecular dynamics simulations demonstrates that the solvation dynamics at the molecular level is strongly influenced by this small structural difference. The effective timescales for solvent migration triggered by ionization are mainly controlled by the efficiency of the kinetic energy redistribution rather than the shape of the potential energy surface. This approach provides a fundamental understanding of protein hydration and may help to design functional molecules in solution with tailored properties.


 Please wait while we load your content...
Please wait while we load your content...