Correlation between collective and molecular dynamics in pH-responsive cyclodextrin-based hydrogels
Abstract
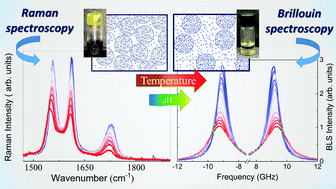
UV Raman and Brillouin light scattering (BLS) experiments have been used in this study to explore the complex phase change behavior occurring in pH-responsive polysaccharide hydrogels as a function of temperature. Due to the different physical quantities measured by the two techniques, the joint analysis of Raman and BLS spectra has provided an unprecedented large-scale characterization of the molecular rearrangements and of the different kinds of hydrophilic and hydrophobic interactions that cooperate to determine the phase transformation observed in these hydrogels during the heating of the gel. As the main result, the analysis of the Raman and BLS spectra showed the existence of a correlation between the local (molecular) and collective properties of the gels during the phase transformation undergone by the system, which is markedly triggered by pH. The joint set of experimental results suggests a model according to which the mechanism of pH dependence in the hydrogels under investigation is dominated by the interactions involving the hydrophobic parts of the polymer skeleton, whereas the solvation process observed under heating of the gels is driven by the progressive distancing of the polymer domains among them, as monitored by the Brillouin sound velocity.


 Please wait while we load your content...
Please wait while we load your content...