Critical universality and asymmetry of ionic solution {iodobenzene + 1-decyl-3-methyl-imidazolium bis(trifluorosulfonyl)imide}†
Abstract
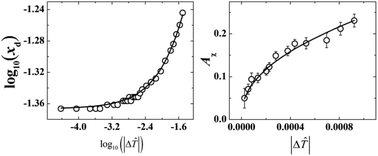
The liquid–liquid coexistence curve, heat capacity in the critical and noncritical regions, and the turbidity in critical one-phase and two-phase regions of the binary solution {iodobenzene + 1-decyl-3-methylimidazolium bis(trifluorosulfonyl)imide ([C10mim][NTf2])} have been precisely measured. From the data collected in the critical region, the critical exponents α, β, γ and ν, as well as the universal critical amplitude ratios RB and X were obtained and were shown to agree well with their theoretical values for the 3D-Ising universality class, which further confirmed the 3D-Ising criticality of ionic solutions even in a very low relative permittivity solvent. The coulombic character of the studied system was suggested by the small values of the RPM reduced critical temperature and density. Furthermore, it was found that both the asymmetric behaviors of the diameter of the coexistence curve and the osmotic compressibility could be well described using the complete scaling theory.


 Please wait while we load your content...
Please wait while we load your content...