The sensitivity of folding free energy landscapes of trpzips to mutations in the hydrophobic core†
Abstract
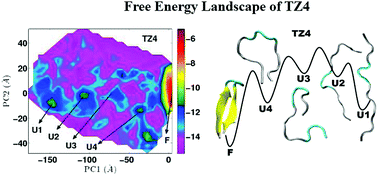
The sensitivity of the stability of folded states and free energy landscapes to the differences in the hydrophobic content of the core residues has been studied for the set of 16-residue trpzips, namely, Trpzip4, Trpzip5 and Trpzip6. The combination of principal component analysis and different secondary structure order metrics as reaction coordinates has been used to characterize and identify all the underlying attractive basins corresponding to the folded and the unfolded states for each trpzip at 300 K. Our results reveal that even a single mutation in the hydrophobic core perturbs the stability of the folded peptide and the conformational preferences for the partially folded and unfolded states significantly, leading to concomitant alterations in the free energy landscape of trpzips. Trpzip4 is observed to have the most rugged and variegated free energy landscape with occurrence of four metastable unfolded states in addition to the folded native state. In contrast, Trpzip5 and Trpzip6 are characterized by two such metastable states. The order metrics pertaining to the rigidity of the turn residues and the distances between the side chains of the hydrophobic core residues have been found to be most revealing to understand the degree of discrimination among the folded states of different peptides in addition to the unfolded states. Our results suggest that both turn propensity and hydrophobic interactions influence the thermodynamics of the folding pathways of trpzips. The implications of the sequence dependent response of amino acids, effect of aromatic stacking interactions and packing of protein's interior for shaping the free energy landscape of the peptides have been highlighted.


 Please wait while we load your content...
Please wait while we load your content...