Kinetics and branching fractions of the hydrogen abstraction reaction from methyl butenoates by H atoms†
Abstract
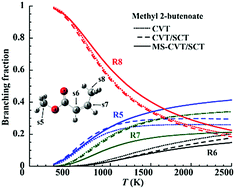
In order to explore the hydrogen abstraction reaction kinetics of unsaturated methyl esters by hydrogen atoms, we selected two molecules for study, in particular methyl 3-butenoate and methyl 2-butenoate, whose C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds are at different locations. We first determined an accurate and efficient electronic structure method for the investigation by considering eight hydrogen abstraction reactions and comparing their barrier heights and reaction energies computed using several exchange–correlation density functionals to those obtained from CCSD(T)-F12a/jun-cc-pVTZ coupled cluster calculations. In this way, we found the M06-2X/ma-TZVP method to have the best performance with a mean unsigned deviation from the CCSD(T) calculations of 0.51 kcal mol−1. Based on quantum-chemical calculations by using the M06-2X/ma-TZVP method, we then computed rate constants for 298–2500 K by direct dynamics calculations using multi-structural canonical variational transition state theory including tunneling by the multi-dimensional small-curvature tunneling approximation (MS-CVT/SCT). The computed transmission coefficients were compared with those obtained using the zero-curvature tunneling (ZCT) and one-dimensional Eckart tunneling (ET) approximations. We employed the multi-structural torsional method (MS-T) to include the multiple-structure and torsional potential anharmonic effects. The results show that the variational recrossing transmission coefficients range from 0.6 to 1.0, and the multi-structural torsional anharmonicity introduces a factor of 0.5–2.5 into the rate constant, while the tunneling transmission coefficients obtained by SCT can be as large as 17.4 and differ considerably from those determined by the less accurate ZCT and ET approximations. In addition, independent of the location of the C
C double bonds are at different locations. We first determined an accurate and efficient electronic structure method for the investigation by considering eight hydrogen abstraction reactions and comparing their barrier heights and reaction energies computed using several exchange–correlation density functionals to those obtained from CCSD(T)-F12a/jun-cc-pVTZ coupled cluster calculations. In this way, we found the M06-2X/ma-TZVP method to have the best performance with a mean unsigned deviation from the CCSD(T) calculations of 0.51 kcal mol−1. Based on quantum-chemical calculations by using the M06-2X/ma-TZVP method, we then computed rate constants for 298–2500 K by direct dynamics calculations using multi-structural canonical variational transition state theory including tunneling by the multi-dimensional small-curvature tunneling approximation (MS-CVT/SCT). The computed transmission coefficients were compared with those obtained using the zero-curvature tunneling (ZCT) and one-dimensional Eckart tunneling (ET) approximations. We employed the multi-structural torsional method (MS-T) to include the multiple-structure and torsional potential anharmonic effects. The results show that the variational recrossing transmission coefficients range from 0.6 to 1.0, and the multi-structural torsional anharmonicity introduces a factor of 0.5–2.5 into the rate constant, while the tunneling transmission coefficients obtained by SCT can be as large as 17.4 and differ considerably from those determined by the less accurate ZCT and ET approximations. In addition, independent of the location of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, the dominant hydrogen abstraction reactions occur at the allylic sites.
C double bond, the dominant hydrogen abstraction reactions occur at the allylic sites.


 Please wait while we load your content...
Please wait while we load your content...