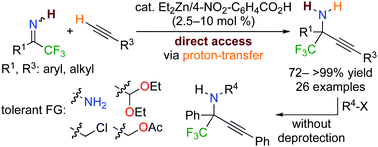
Direct access to N-unprotected tetrasubstituted propargylamines via direct catalytic alkynylation of N-unprotected trifluoromethyl ketimines†
Abstract
Direct catalytic alkynylation of N-unprotected trifluoromethyl ketimines is reported for the first time. A combination of catalytic amounts of diethylzinc and carboxylic acids promoted the reactions under proton-transfer conditions, allowing an unprecedented direct access to N-unprotected α-tetrasubstituted primary amines without additional deprotection steps.


 Please wait while we load your content...
Please wait while we load your content...