Concentration-dependent circularly polarized luminescence (CPL) of chiral N,N′-dipyrenyldiamines: sign-inverted CPL switching between monomer and excimer regions under retention of the monomer emission for photoluminescence†
Abstract
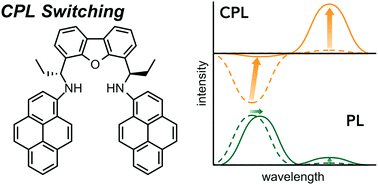
Herein, an unprecedented switching of circularly polarized luminescence (CPL) is described for chiral 4,6-bis(1-(pyren-1-ylamino)propyl)dibenzo[b,d]furan (1). The CPL band of chiral diamine 1, which contains two pyrene rings, can be switched between the monomer and excimer emission regions under concomitant inversion of the handedness, simply by changing the concentration of the fluorophore. In contrast, the maximum photoluminescence (PL) intensity is always observed in the monomer region, regardless of the concentration. The reversal of the intensity ratio of monomer and excimer emission between PL and CPL was attributed to a stronger CPL (|gem| = ∼3–4 × 10−3) contribution from the minor excimer component, which should exhibit an efficient chiral environment around the dimeric pyrenes.


 Please wait while we load your content...
Please wait while we load your content...