Evidence of robust participation by an equatorial 4-O group in glycosylation on a 2-azido-2-deoxy-glucopyranosyl donor†
Abstract
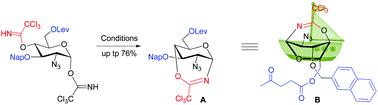
Although there are numerous claims of remote group participation leading to the synthesis of the expected glycosides with improved anomeric geometry outcome in glycosylation, there is still a lack of enough strong evidence and this has led to a long-term debate, particularly for equatorial 4-O group participation. In this work, we were able to synthesize and isolate a stable seven-membered trichlorooxazepine ring bridging intermediate with a high yield by employing a 2-azido-2-deoxy-glucopyranosyl donor, which provides strong evidence to support the putative participation of the equatorial 4-O group in glycosylation.


 Please wait while we load your content...
Please wait while we load your content...