Development of catalytic deacylative alkylations (DaA) of 3-acyl-2-oxindoles: total synthesis of meso-chimonanthine and related alkaloids†
Abstract
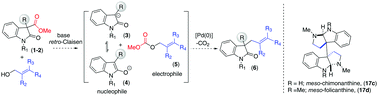
We present an effective deacylative alkylation strategy for the construction of a variety of 2-oxindoles bearing an all-carbon quaternary center at the pseudobenzylic position. A wide variety of products with quaternary centers could be accessed by employing simple Pd(0) catalysis under mild reaction conditions. Importantly, the same strategy works equally well for the dimeric 2-oxindole system, furnishing products with a vicinal quaternary center in favour of meso-isomer as the major product. Eventual application to the total syntheses of meso-chimonanthine and meso-folicanthine very well demonstrates the synthetic potential of this strategy.


 Please wait while we load your content...
Please wait while we load your content...