An atom- and pot-economical consecutive multi-step reaction approach to polycyclic aromatic hydrocarbons (PAHs)†
Abstract
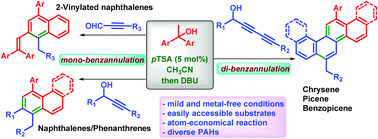
A novel one-pot benzannulation reaction has been developed for the synthesis of substituted polycyclic aromatic hydrocarbons (PAHs) from the direct coupling of propargylic aldehydes/alcohols with 1,1-diarylethanol through an atom-economical uninterrupted three/four-step reaction sequence under mild and metal-free reaction conditions. The strategy involves an acid-catalyzed dehydration and carbon–carbon bond formation followed by DBU-promoted cycloisomerization. Naphthalene and phenanthrene were obtained via mono-benzannulation, and chrysene, picene and benzopicene were obtained involving consecutive di-benzannulation reactions in good yields starting from easily accessible starting materials.


 Please wait while we load your content...
Please wait while we load your content...