Hydrogen-adduction to open-shell graphene fragments: spectroscopy, thermochemistry and astrochemistry
Abstract
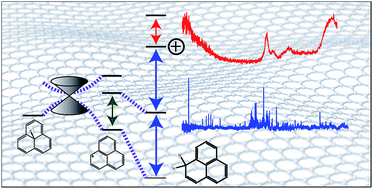
We apply a combination of state-of-the-art experimental and quantum-chemical methods to elucidate the electronic and chemical energetics of hydrogen adduction to a model open-shell graphene fragment. The lowest-energy adduct, 1H-phenalene, is determined to have a bond dissociation energy of 258.1 kJ mol−1, while other isomers exhibit reduced or in some cases negative bond dissociation energies, the metastable species being bound by the emergence of a conical intersection along the high-symmetry dissociation coordinate. The gas-phase excitation spectrum of 1H-phenalene and its radical cation are recorded using laser spectroscopy coupled to mass-spectrometry. Several electronically excited states of both species are observed, allowing the determination of the excited-state bond dissociation energy. The ionization energy of 1H-phenalene is determined to be 7.449(17) eV, consistent with high-level W1X-2 calculations.


 Please wait while we load your content...
Please wait while we load your content...