Simultaneous construction of two linkages for the on-surface synthesis of imine–boroxine hybrid covalent organic frameworks†
Abstract
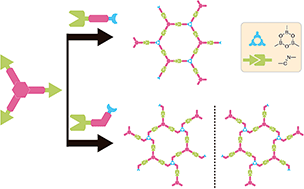
The orthogonality between the Schiff base reaction and the boronic acid dehydration reaction is explored during the on-surface synthesis process. By activating the above two reactions in one-step and employing asymmetrical substituted monomers and the 3-fold symmetric monomer 1,3,5-tris(4-aminophenyl)benzene (TAPB), highly ordered imine–boroxine hybrid single-layered covalent organic frameworks (sCOFs) have been successfully constructed on HOPG by a gas–solid interface reaction method and characterized by scanning tunnelling microscopy (STM). In particular, the reaction between the meta-substituted monomer and TAPB generates sCOFB with a windmill structure, which is the first sCOF with surface chirality so far reported. The demonstration of the one-step synthesis of multiple linkages to form sCOFs can further enlarge the sCOF family and expand the design routes for functional 2D organic nanomaterials.

 Please wait while we load your content...
Please wait while we load your content...