CO/O2 assisted oxidative carbon–carbon and carbon–heteroatom bond cleavage for the synthesis of oxosulfonates from DMSO and olefins†
Abstract
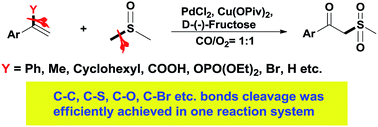
Selective carbon–carbon and carbon–heteroatom bond cleavage was achieved in a one reaction system. With this strategy a novel Pd/Cu-catalyzed aerobic oxidative oxosulfonation of olefins with DMSO has been developed. Preliminary mechanistic investigations indicated that CO/O2 assisted the bond cleavage and the leaving groups from the starting materials were trapped by O2 and underwent a hydroxylation process.


 Please wait while we load your content...
Please wait while we load your content...