Tethered tertiary amines as solid-state n-type dopants for solution-processable organic semiconductors†
Abstract
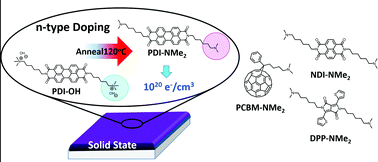
A scarcity of stable n-type doping strategies compatible with facile processing has been a major impediment to the advancement of organic electronic devices. Localizing dopants near the cores of conductive molecules can lead to improved efficacy of doping. We and others recently showed the effectiveness of tethering dopants covalently to an electron-deficient aromatic molecule using trimethylammonium functionalization with hydroxide counterions linked to a perylene diimide core by alkyl spacers. In this work, we demonstrate that, contrary to previous hypotheses, the main driver responsible for the highly effective doping observed in thin films is the formation of tethered tertiary amine moieties during thin film processing. Furthermore, we demonstrate that tethered tertiary amine groups are powerful and general n-doping motifs for the successful generation of free electron carriers in the solid-state, not only when coupled to the perylene diimide molecular core, but also when linked with other small molecule systems including naphthalene diimide, diketopyrrolopyrrole, and fullerene derivatives. Our findings help expand a promising molecular design strategy for future enhancements of n-type organic electronic materials.
- This article is part of the themed collections: Global Energy Challenges: Solar Energy, Top 50 Articles of 2016: Energy and Catalysis and Top 50 Articles of 2016: Materials Chemistry and Nanoscience

 Please wait while we load your content...
Please wait while we load your content...