Additive effect of fluoroethylene and difluoroethylene carbonates for the solid electrolyte interphase film formation in sodium-ion batteries: a quantum chemical study†
Abstract
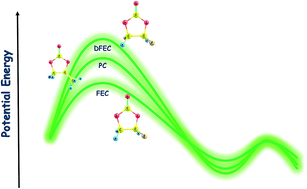
Sodium (Na)-ion batteries (NIBs) are attracting noticeable interest in recent years as post lithium (Li)-ion batteries (LIBs) due to the “unlimited” abundance of sodium in the Earth's crust. To improve the performance of secondary batteries, solid electrolyte interface (SEI) film-forming additives in electrolyte solutions are widely used in general and fluoroethylene carbonate (FEC) additive is known to increase the NIB performance, while difluoroethylene carbonate (DFEC) is inefficient in spite of it being a similar molecule substituted by only one fluorine atom. Such fine behavior of electrolyte additives in the NIBs is not thoroughly understood, i.e., the FEC–DFEC mystery. In this article, to understand the chemical roles of these additives atomistically, we have investigated the reduction decomposition mechanism of three complexes, i.e., Na+ coordinated propylene carbonate (Na+–PC), Na+–FEC and Na+–DFEC using sophisticated computational methods. The activation energy barriers of Na+–PC, Na+–FEC and Na+–DFEC are found in the gas phase as 11.696, 7.050, and 15.125 kcal mol−1 respectively, while, in solution phase (PC as solvent of dielectric constant 64.4), those barriers become 9.993, 6.594 and 14.012 kcal mol−1 respectively. Because the lower activation barrier of FEC facilitates the faster decomposition than those of PC and DFEC, it is clearly expected that the faster decomposition of FEC than that of PC must be one of the important factors necessary to improve the SEI film formation. However, DFEC reductive decomposition is slower than those of PC and FEC due to its higher activation energy barrier. Another important factor is that the DFEC decomposition does not yield NaF, whereas FEC does on its decomposition. This could also be a key reason why FEC is a better additive than DFEC.

 Please wait while we load your content...
Please wait while we load your content...