Purification, properties and application of a collagenolytic protease produced by Pseudomonas sp. SUK
Abstract
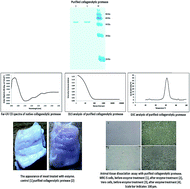
The extracellular collagenolytic protease produced by Pseudomonas sp. SUK was purified to homogenecity by ammonium sulfate precipitation followed by DEAE-cellulose anion exchange chromatography. The enzyme was purified by 15.40 fold with 26.80% recovery and its molecular mass was found to be 58.6 kDa by SDS-PAGE. The optimum temperature and pH for the enzyme were 60 °C and 8.0, respectively. The purified enzyme was stable over a wide pH and temperature range and it was able to degrade various types of collagen. The Km and Vmax of the enzyme was 1.05 ± 0.09 mg ml−1 and 6.03 ± 0.52 × 10−4 mol l−1 min−1, respectively. EDTA, Fe3+, Hg2+, SDS, methanol and iso-propyl alcohol inhibited >25% enzyme activity whereas Zn2+, Ba2+, Ca2+, Tween 80, toluene and n-hexane were found to be good enhancers. Biophysical characterization revealed that the enzyme is 68.4% α-helix and 8.32% β-sheet, with hydrodynamic radius of approximately 3.1 nm. Furthermore the enzyme has a negative charge at pH 7.5 with a zeta potential value of −28.7 mV and Tm 62.3 °C ± 0.02 °C. This study assumes that the collagenolytic protease purified from Pseudomonas sp. SUK could be potentially exploited for meat tenderization at reduced temperatures as well as in animal tissue cultures as a tissue dissociating and cell dislodging agent.

 Please wait while we load your content...
Please wait while we load your content...