α,ω-Bis(trialkoxysilyl) difunctionalized polycyclooctenes from ruthenium-catalyzed chain-transfer ring-opening metathesis polymerization†
Abstract
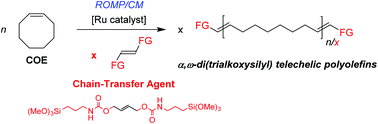
The ring-opening metathesis polymerization/cross-metathesis (ROMP/CM) of cyclooctene (COE) using bis(trialkoxysilyl)alkenes as chain-transfer agents (CTAs) and Ru catalysts to afford difunctionalized polyolefins is reported. The formation of α,ω-bis(trialkoxysilyl) telechelic polycycloolefins (DF) with controlled molar mass values takes place quite selectively (>90 wt%), along with minor amounts of cyclic non-functionalized polymers (CNF), as evidenced by NMR, MALDI-ToF MS, SEC analyses and fractionation experiments. The nature of the CTA and catalyst influenced much the efficiency and selectivity of the reaction. (MeO)3SiCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Si(OMe)3 (2) and (MeO)3Si(CH2)3NHC(O)OCH2CH
CHCH2Si(OMe)3 (2) and (MeO)3Si(CH2)3NHC(O)OCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2OC(O)NH(CH2)3Si(OMe)3 (5) proved to be the most efficient CTAs in terms of reactivity, catalyst productivity and selectivity towards DF. Diurethane CTA 5 is easily prepared, and can also be conveniently generated in situ during the ROMP/CM. Grubbs’ 2nd-generation catalyst (G2) and Hoveyda–Grubbs's catalyst (HG2) afforded the best compromise in terms of selectivity and productivity, with turnover numbers of up to 95 000 mol(COE) mol(Ru)−1 and 5000 mol(CTA) mol(Ru)−1.
CHCH2OC(O)NH(CH2)3Si(OMe)3 (5) proved to be the most efficient CTAs in terms of reactivity, catalyst productivity and selectivity towards DF. Diurethane CTA 5 is easily prepared, and can also be conveniently generated in situ during the ROMP/CM. Grubbs’ 2nd-generation catalyst (G2) and Hoveyda–Grubbs's catalyst (HG2) afforded the best compromise in terms of selectivity and productivity, with turnover numbers of up to 95 000 mol(COE) mol(Ru)−1 and 5000 mol(CTA) mol(Ru)−1.

 Please wait while we load your content...
Please wait while we load your content...