Multiblock thermoplastic elastomers via one-pot thiol–ene reaction†
Abstract
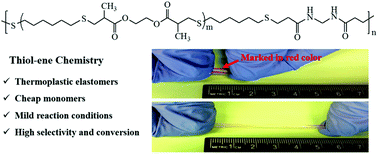
We report a facile approach to designing multiblock thermoplastic elastomers using a two-step thiol–ene polyaddition reaction. It is based on the utilization of intermolecular hydrogen bonding of widely available and cost-effective monomer of N,N′-methylenebis(acrylamide) (MBAm) as physical cross-links. Thiol-terminated “soft” prepolymers were first prepared using ethylene glycol dimethacrylate (EGDMA) and an excess of 1,6-hexanedithiol (HDT); subsequently, the thiol-terminated prepolymers were further reacted with MBAm as a chain-extension reaction to yield the multiblock thermoplastic elastomers. The prepolymers with oligo(ethylene glycol) segments had a low glass-transition temperature, acting as elastic “soft” blocks; while MBAm units could form up to 4 hydrogen bonds that serve as physical networks to endow the elasticity to multiblock polymers. Proton nuclear magnetic resonance spectroscopy and gel permeation chromatography indicated the occurrence of the two-step thiol–ene reactions. The reaction kinetics of thiol–ene reactions was found to be highly dependent on the molecular weights of monomers. The first thiol–ene reaction of EGDMA and HDT could reach >90% conversion of both monomers within 5 min; while the kinetics of the second chain extension reaction was relatively slow and it took approximately 7 h to reach 90% conversion. The formation of the intermolecular hydrogen bonding between amide groups of MBAm units was confirmed by variable-temperature Fourier transform infrared spectroscopy and differential scanning calorimetry. The viscoelasticity and elasticity of the thermoplastic elastomers were found to be largely determined by the content of MBAm. With a molar ratio of 15% MBAm relative to EGDMA, the maximum elongation at break of elastomers reached >400%. Our synthetic method has the advantages of mild reaction conditions, high conversion and adjustable mechanical properties of elastomers; additionally, it does not involve heavy syntheses and expensive monomers/catalysts. Our findings conceivably stand out as a new tool to synthesize and engineer thermoplastic elastomers using the combination of thiol–ene chemistry and supramolecular interaction.

 Please wait while we load your content...
Please wait while we load your content...