Highly enantioselective synthesis of spirocyclopropyloxindoles via a Rh(ii)-catalyzed asymmetric cyclopropanation reaction†
Abstract
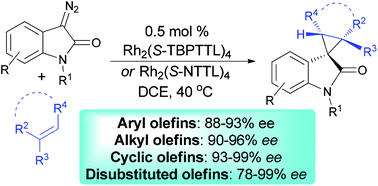
A chiral dirhodium(II) carboxylate complex catalyzed enantioselective cyclopropanation reaction of N-Boc diazooxindoles with olefins, including aryl, alkyl, cyclic, and disubstituted olefins, under mild conditions is described. This reaction provides complementary access to the corresponding chiral spirocyclopropyloxindole products in good to high yields with good to excellent enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...