Total synthesis of (±)-ganocins B and C†
Abstract
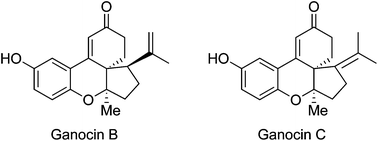
The first total synthesis of structurally unique polycyclic phenolic meroterpenoids, ganocins B and C is reported. The synthesis features gold-catalyzed intramolecular cascade cyclization to construct the C/D ring bearing an angular methyl group, diastereoselective Michael addition, and acid-mediated one-pot Robinson cyclization/deprotection/isomerization.

 Please wait while we load your content...
Please wait while we load your content...